Key Benefits of Catalysts
Catalysts play a vital role in various industries, enabling efficient and cost-effective production processes.
Goodfellow's specialist materials allows you to:
- Access catalytic materials with high surface areas, maximizing the number of active sites available for reactions: for faster reaction rates for process efficiency and productivity, lower operational temperatures to save energy or avoid thermal degradation and improve the desired reaction path
- Customize the properties of your catalytic material: by choosing the right support materials for performance, the amount of precious metal deposited and the promotor / inhibitor addition for the targeted reactions needed
There are numerous possibilities to choose the most suitable catalyst based on your specific needs and the desired reaction, some of these advantages include:
- Accelerated reaction rates: Catalytic materials enable faster chemical reactions, significantly reducing production time
- Process efficiency: Catalysts can be tailored to promote specific reactions and selectively produce desired products
- Emissions reduction: Catalysis plays a crucial role in reducing harmful emissions from chemical processing or combustion
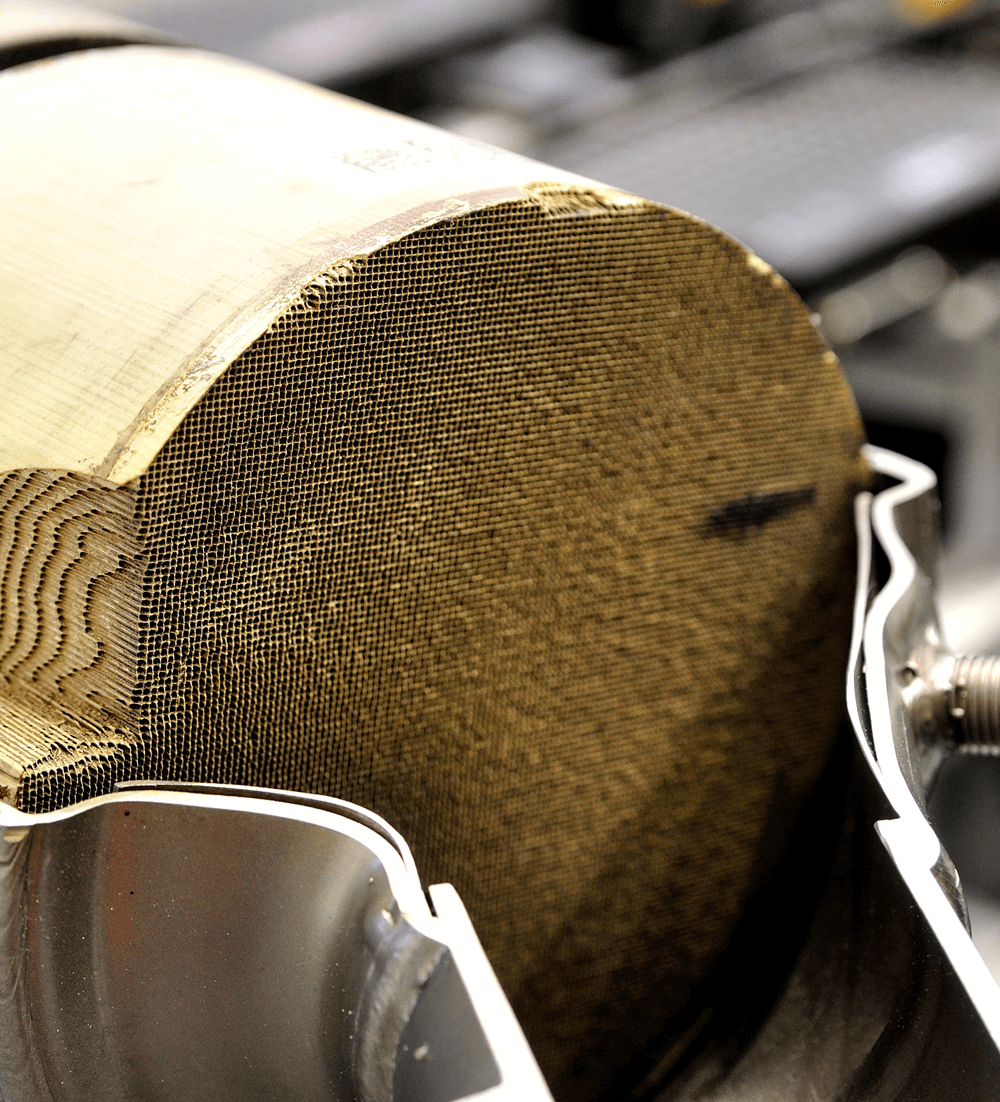

Catalytic Materials Delivered Worldwide
Whether you need catalytic materials for chemical synthesis or to help lower emissions from chemical processing or combustion, Goodfellow is a leading supplier of catalysts, including homogeneous, heterogeneous, and biocatalyst, focused on quality and reliability. Available for delivery anywhere in the world.
In addition to supplying existing catalysts, both common and rare, our team of experts can partner with you to research and develop efficient and optimized catalytic processes.


Catalytic Materials Product Properties
We offer a wide range of technical solutions and our industry leading experts are on hand to help with your specifications.


Palladium
This rare metal is prized for its stability. An excellent catalyst for the absorption of oxygen atoms and oxidation of highly poisonous carbon monoxide.


Platinum
Sometimes combined with the other metals. Platinum as a photocatalyst – with platinum nanoparticles being used to oxidize carbon monoxide, nitrous oxides, and for water-splitting applications in hydrogen generation.

Nickel
One of the most famous nickel-based catalysts is Raney nickel, a heterogenous hydrogenation catalyst that is a nickel-aluminum alloy. This is used in hydrogenation reactions, including the production of cyclohexane from benzene.


Catalytic Supports
Ceria (cerium oxide) and alumina (aluminum oxide) are the most common catalytic supports. They provide a scaffold onto which another material can be coated or grown to improve the surface area and catalytic performance.


Metal Oxides
A diverse class of catalyst, with titanium dioxide and zinc oxide being popular. Often used in a variety of forms, from thin films to nanoparticle structures. Their high surface area and reactivity make them highly effective.

Electrode Materials
Here, reactions can be controlled by applying voltages to electrodes. Platinized electrodes are a popular choice for heterogenous catalysis, but there are several homogenous electrocatalysts that can facilitate a chemical reaction.


Nanoparticles
With inherently large surface areas, their electrochemical and optical properties are size dependent. They can be either adhered to electrodes or used in solution as a catalyst.

Nanomaterials
Normally a more affordable option in the longer term than materials like platinum and palladium. Hence their popularity in the automotive industry as the costs for traditional catalysts used continue to increase.
Applications
Catalysts find extensive applications in a wide range of industries including:

Chemical Synthesis
Catalytic materials, such as transition metal complexes, are used to facilitate various chemical reactions, including oxidation, reduction, and hydrogenation. For example, platinum-based catalysts are widely used in the synthesis of pharmaceutical compounds. They enable the efficient conversion of organic molecules, leading to the production of life-saving medications.


Emission Control
Catalytic converters in automobiles are a prime example of catalysis in emissions control. These devices utilize catalytic materials, such as platinum, palladium, and rhodium, to convert harmful pollutants, such as carbon monoxide, nitrogen oxides, and unburned hydrocarbons, into less harmful substances. These converters reduce the environmental impact of vehicle emissions, contributing to improved air quality and public health.


Petrochemical Industry
The petrochemical industry heavily relies on catalytic materials for processes like hydrocracking, reforming, and isomerization. These reactions are essential for producing fuels, lubricants, and other valuable petrochemical products. For instance, zeolites, a type of crystalline aluminosilicate catalyst, are widely used in the petrochemical industry. They exhibit excellent selectivity and stability, making them ideal for hydrocarbon conversion and refining processes.


Energy Conservations
Catalytic materials play a crucial role in energy conversion processes. For instance, in fuel cells, catalysts facilitate the electrochemical reactions that convert chemical energy into electrical energy. Platinum-based catalysts are commonly used in the cathode of proton exchange membrane fuel cells, enabling sustainable energy generation. Moreover, catalytic materials are instrumental in hydrogen production through water splitting. These materials enhance the efficiency of the reaction, enabling the generation of clean and renewable hydrogen fuel.
What's New