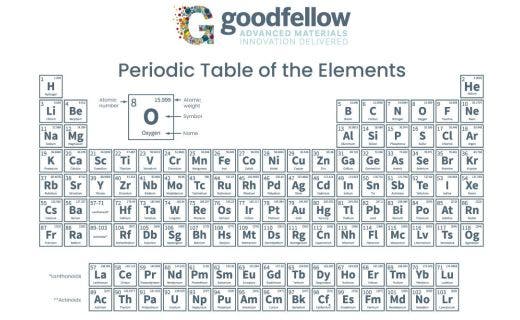
The periodic table is a chart that shows all of the elements. Elements are the materials that make up everything in the world. The smallest possible piece of an element is called an atom, and all of the elements on the periodic table are arranged based on the properties of their atoms. This helps scientists to understand the elements better just by looking at the periodic table.
Why Is the Table "Periodic"?
The periodic table is called that because the elements are arranged in a way that makes a pattern. The word "periodic" means "repeating in a cycle," and that's what some of the traits of the elements do. The periods on the periodic table are the rows; each row is a different period.
As you go across the table from left to right, each element has one more electron in it than the last one. These electrons, which are the tiny particles that make up electricity, are inside of each atom, but they can only sit in certain spaces, called shells. Every period of the periodic table contains elements that all have the same number of shells. For instance, in the first row of the periodic table, there are only two elements, and they both have one shell. The next row, or period, has eight elements, and they all have two shells. Some shells are bigger than others, so they can hold more electrons; this explains why some rows have fewer elements than others do.
Why Are There Big Gaps in the Rows at the Top of the Table?
The elements at the top of the periodic table aren't right next to each other because the periodic table isn't just arranged into periods: It's also arranged into groups. The groups are the different columns. All of the elements in each group share similar properties because they have the same number of electrons in their outer shell. The first group has elements that have one electron in their outer shell, which makes these elements very likely to react with other elements. The last group, all the way on the right, has elements with completely full electron shells. These elements don't usually react with anything.
What Do the Letters Mean?
Each box contains a one- or two-letter abbreviation that's used for that element. For example, "H" is hydrogen, "He" is helium, "C" is carbon, and "O" is oxygen. Some versions of the periodic table will also spell out the full name of each element underneath the abbreviation.
What Are the Numbers in Each Box?
The number in each box is that element's atomic number. The atomic number is the number of protons that an atom has. Protons are positively charged particles that balance out the negatively charged electrons. Atoms usually have the same number of protons and electrons, so the atomic number also usually tells you the number of electrons in an atom. You might see more than one number in each box on a periodic table; if there's another, smaller number, it's the atomic weight. The atomic weight is the average weight of one atom of that element.
Who Came Up With the Periodic Table?
A Russian scientist named Dmitri Mendeleev created the periodic table in 1869. He did such a good job organizing the table that he could actually use it to predict what elements would be like before they were discovered. Mendeleev left holes in his table wherever none of the known elements fit, and these holes were later filled in as new elements were found.
Fun Facts About the Periodic Table
- The periodic table just keeps growing! We add on new boxes and even whole new rows as scientists find new elements.
- These days, we don't really discover elements anymore, as we've already found all of the ones that are naturally present in the world. New elements are created in science labs. To do this, they use machines that shoot two atoms toward each other at really high speeds. When the atoms crash into each other, they might fuse together and create a new atom of an element we've never seen before.
- Whoever finds a new element usually gets to name it. Elements have been named after famous people, places, and characters from mythology.
- The most common elements on Earth are hydrogen, helium, oxygen, and carbon.
- Earth's core is made mostly of nickel and iron.
- Element 101 is named after Mendeleev: It's called mendelevium.
- Argentina got its name from an element. The country is known for its silver, and "silver" in Latin is "argentum," from which the element gets its abbreviation, "Ag."